Mob/ Whatsapp/Wechat: +86 138 806 76215 | Email: [email protected] / [email protected]
 Rapid Plasmid DNA Extraction Mini Kit adopts the latest invention of special neutralization solution Buffer N8, which allows users to quickly extract up to 50 g high purity plasmid DNA from 1~5 ml of bacterial culture (LB medium) in 8 minutes.
Rapid Plasmid DNA Extraction Mini Kit adopts the latest invention of special neutralization solution Buffer N8, which allows users to quickly extract up to 50 g high purity plasmid DNA from 1~5 ml of bacterial culture (LB medium) in 8 minutes.  The plasmid DNA obtained is suitable for molecular biology experiments such as restriction enzyme digestion, sequencing, library screening, ligation and transformation, in vitro transcription and translation, and transfection of robust cells.
The plasmid DNA obtained is suitable for molecular biology experiments such as restriction enzyme digestion, sequencing, library screening, ligation and transformation, in vitro transcription and translation, and transfection of robust cells.Rapid Plasmid DNA Mini Kit Cat. No. | 5 preps 1005005 | 50 preps 1005050 | 250 preps 1005250 |
Spin columns 2 ml collection tubes RNase A Buffer Ⅰ Buffer Ⅱ Buffer N8 Buffer W2 (concentrate) Buffer E Handbook | 5 pcs 5 pcs * 1.5 ml 1.5 ml 2 ml 2.5 ml 0.6 ml 1 | 50 pcs 50 pcs 28 ml 14 ml 14 ml 20 ml 25 ml 6 ml 1 | 250 pcs 250 pcs 140 ml 70 ml 70 ml 100 ml 65 ml×2 30 ml 1 |
* RNase A from 5 preps has been added to Buffer I.
The Rapid Plasmid DNA Mini Kit adopts the latest invention of special neutralization solution Buffer N8, which allows users to quickly extract up to 50 mg high purity plasmid DNA from 1~5 ml of bacterial culture (LB medium) in 8 minutes. The plasmid DNA obtained is suitable for molecular biology experiments such as restriction enzyme digestion, sequencing, library screening, ligation and transformation, in vitro transcription and translation, and transfection of robust cells. If purified plasmid DNA is used for transfection of sensitive cells, choose the Endo-free Plasmid DNA Mini Kit (Cat. No. 1009050), Endo-free Plasmid DNA Double Mini Kit (Cat. No. 1006050) or Endo-free Plasmid DNA Midi Kit (Cat. No. 1016025)。
Product Storage and Expiry Date
2. Add RNase A Buffer I to store at 2~8 ℃. If stored for more than 6 months, RNase A should be re-added in Buffer I to a final concentration of 100 μg/ml.
3. If other reagents and articles are stored at room temperature (0~30 ℃), they can maintain no significant change in performance within two years; If the product is stored at 2~8 ℃, the validity period of the product can be extended to more than two years (the product stored at 2~8 ℃ should be restored to room temperature before use).
Equipment and Reagents to Be Supplied by User
1. Absolute ethanol
2. Buffer W1 may needed.
3. 1.5 ml and 2 ml microcentrifuge tubes, pipette, and tips
4. Protective equipment such as disposable latex gloves and paper towels
5. Microcentrifuge(s) (with rotor for 1.5 ml and 2 ml microcentrifuge tubes)
6. Vortexer
Rapid Plasmid DNA Mini Kit is most suitable for use in experiments such as plasmid construction, clone identification, and sequencing, these experiments usually uses high-copy plasmids, and the host bacteria are DH5α or other endA- bacterial. This product can extract plasmid DNA from 1-5 ml of overnight bacterial culture (LB medium) and the operation is very simple and fast.
Rapid Plasmid DNA Mini Kit is suitable for the isolation of plasmid DNA from Gram-negative bacteria(such as E. coli). If it is necessary to isolate plasmid DNA from Gram-positive bacteria, please first break the wall of the bacteria with lysozyme, refer to "Appendix 1: Extraction of Gram-positive Bacterial Plasmid DNA" on page 12 for details.
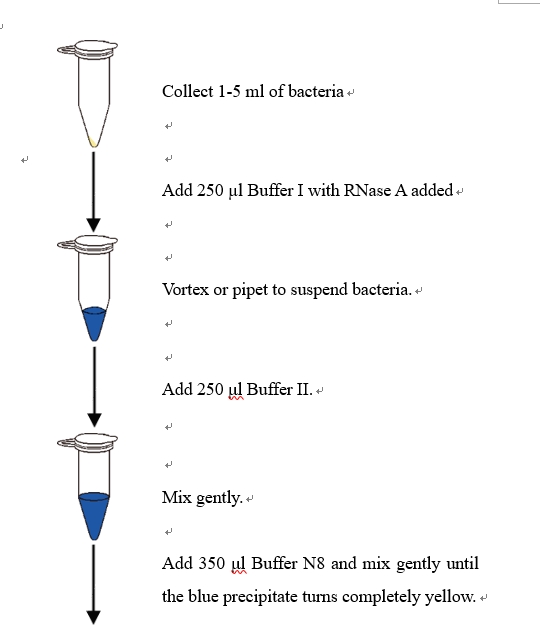
1.Harvest 1-5 ml overnight cultures of bacteria cells in LB (Luria-Bertani) medium by centrifuging at 12,000 rpm for 30 s, discard the medium. Fully suspend pelleted bacterial cells in 250 μl Buffer I with addition of RNase A.
* The pellet could be suspended completely by vortexing or pipetting up and down until no cell clumps left. Otherwise it would seriously affect the final plasmid DNA yield.
2. Add 250 μl Buffer II and mix thoroughly by gently inverting the tube 4 - 6 times.
* Before using Buffer II, make sure that no visible precipitate exists in the solution. After applying Buffer II, tighten the lid to avoid prolonged contact with air.
* Do not vortex, as this will result in shearing of genomic DNA.
* The solution should be viscous and slightly clear when the bacterial is lysed sufficiently;if too much bacteria were used, continue inverting the tube until the solution becomes viscous and slightly clear.
* Do not allow this step to proceed for more than 5 minutes.
3. Add 350 μl Buffer N8, mix gently and thoroughly by inverting the tube until the color of precipitate changes from blue to light yellow completely.
* Do not vortex, as this will result in shearing of genomic DNA.
* Continue to invert gently 4 - 6 times after the color of precipitate changes from blue to light yellow completely, if the bacteria culture medium used was more than 3 ml.
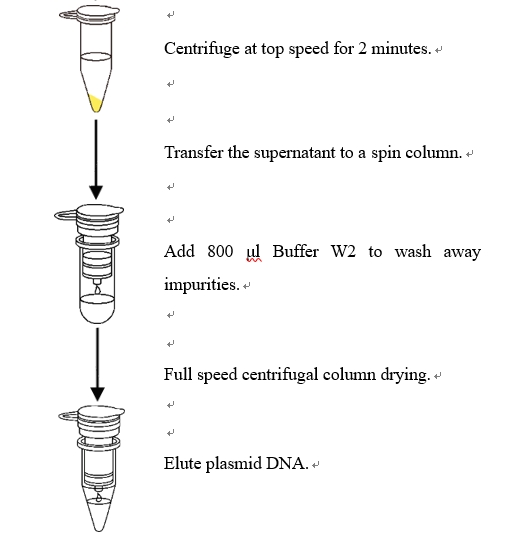
4. Centrifuge at full speed ( ≥12,000 rpm ) for 2 min.
* Prolong the centrifuge time to 5 min if more than 3 ml bacteria culture medium was used.
5. Place a spin column in a 2 ml collection tube (provided), decanting the supernatant from step 4 into the spin column, close the lid and centrifuge at 12,000 rpm for 30 s.
* Decanting the supernatant into the spin column immediately after centrifugation, stay for a long time may cause the precipitate to float, which makes it hard to operate in following procedure.
6. Discard the filtrate. Place the spin column back to the collection tube. Add 800 µl Buffer W2 to the spin column. Closed the lid and centrifuge at 12,000 rpm for 30 s.
* Repeat step 6 is recommend to ensure high quality of plasmid, if plasmid will be used for cell transfection.
* Ensure ethanol has been added into Buffer W2.
* For endA+ host bacteria (e.g. wild-type E.coli, HB101, BL21), washing the spin column by adding Buffer W1 (Order separately, Cat. No. B025-01 ) is necessary to remove trace nuclease activity.
7. Discard the filtrate. Place the spin column back to the collection tube. Centrifuge at full speed ( ≥ 12,000 rpm) for 1 min.
* Do not omit this step, as this will cause problems in downstream applications due to the residual ethanol in the eluate.
* The deionized water can also be used to elute the DNA, but the pH should be ensured at 7.0 - 8.5, otherwise it may affect the elution efficiency.