Mob/ Whatsapp/Wechat: +86 138 806 76215 | Email: [email protected] / [email protected]
 The DNA identification system 2 0A uses five-color fluorescent labeling technology to amplify 19 autosomal S TR loci and one Amel locus at one time.
The DNA identification system 2 0A uses five-color fluorescent labeling technology to amplify 19 autosomal S TR loci and one Amel locus at one time. The concentration of DNA typing standard 9947A in this kit is 1ng/ μl .
The concentration of DNA typing standard 9947A in this kit is 1ng/ μl . suitable for amplification after sample extraction, and can also directly amplify blood stain or saliva spot samples on FTA cards,which is no need to use extraction kit .
suitable for amplification after sample extraction, and can also directly amplify blood stain or saliva spot samples on FTA cards,which is no need to use extraction kit .
The DNA identification system 2 0A uses five-color fluorescent labeling technology to amplify 19 autosomal S TR loci and one Amel locus at one time.
1. After receiving the kits frozen in dry ice or gel ice packs, if they are not used temporarily, please store them below -20°C;
2. After the kit is taken out for use, the pre-reaction components 2.5× PCR reaction buffer II and Taq polymerase II are stored at -20°C, and the remaining pre-reaction reagents are stored at 4°C to avoid repeated freezing and thawing. If the single dosage is small, it is recommended to store at -20°C after aliquoting ;
3. Store the components after the reaction at 4°C, avoid repeated freezing and thawing, and do not touch the reagents before the reaction to avoid contamination.
1. amount of DNA template
The concentration of DNA typing standard 9947A in this kit is 1ng/ μl . Users can properly adjust the amount of template added in the PCR system and the number of cycles in the PCR program according to their own DNA template concentration. If the amount of template is too low, some alleles may be missing, and if the amount of template is too high, it may lead to results beyond the detection range of the genetic analyzer.
2. Shaking and mixing of reagents
In order to obtain the best amplification effect, it is recommended to use the previous 2.5×PCR reaction buffer II and 20A primer mixture to vortex for 10 seconds and then centrifuge at low speed to rule out the uneven concentration of the solution caused by possible tube wall adsorption.
3. Amplification system preparation
Table1: Standard (extraction) amplification system preparation composition
components | 25ul system ( μl ) | 10μl system ( μl ) | |
Master Mix | 2.5× PCR reaction buffer II | 10.0 | 4.0 |
5× 20Aprimer mix | 5.0 | 2.0 _ | |
Taq polymerase II | 0.5 | 0.2 | |
Deionized water | 7.0 | 2.8 | |
template DNA | 2.5 | 1.0 | |
total reaction volume | 25 μl | 10 μl | |
4. Protocol settings
Table2: PCR amplification procedure
pre- denatured | 9 5 °C, 5 minutes |
30 cycles | 9 4 °C, 30 seconds |
60°C, 1 point | |
70 °C, 1 minute | |
final extension | 60°C, 30 points |
Cryopreservation | 15°C |
NOTE: Use an ABI 9700 (Gold Block) cycler and perform amplification in MAX mode.
1. Make sure that the POP4 gel and Buffer of the sequencer are within the validity period ;
2. Mix the 5 -color Matrix spectral calibration reagent and deionized formamide according to the following ratio , vortex and mix well, and dispense into a 96-well plate , 10 μl per well;
Instrument type | 3130 x l | 3500 | 3 500 xl |
5-color Matrix | 8 μl | 2.7 μl | 5 μl |
Deionized formamide | 200μl | 100 μl | 250μl _ |
3. After denaturation at 95°C for 3 minutes , quickly cool at 0°C for 3 minutes;
4. Spectral Calibration Recommended Parameters
4.1. 3130 series: ①Create a Run Module: Name it GoldeneyeG5Matrix, select SPECTRAL for Type, and Spect36_POP4 for Template ; in Run Module Settings: fill in 3 for Injection_Voltage, fill in 10 for Injection_Time, fill in 1500 for Run_Time, and fill in other parameters according to the default values. ②Create Protocol: Name as Golden Eye yeG5, select G5 for Dye Set, and select Golden Eye yeG5Matrix for Run Module ; in Edit Parameter, fill in 2.0 for Lower of Matrix Condition Number Bounds, and fill in 20 for Upper.
4.2. 3500 series: Create Dye Set : Dye Set Name is named GoldenEye G5, Chemistry selects Matrix Standard, Dye Set Template selects G5 Template ; in the Parameters option, Matrix Condition Number Upper Limit is set to 8.0 (adjustable according to the actual situation), and After Scan is set Subtract 200 from the Scan point value of the first fragment peak of the matrix , add 200 to the Scan point value of the largest fragment peak of the matrix in Before Scan, and fill in other parameters with default values.
5. In order to obtain the best correction effect, it is recommended to set the Sensitivity to 0.4, the Minimum Quality Score to 0.95 , and at the same time ensure that the spectral correction peak height is between 750rfu-4000rfu (3 130 series) or 3000rfu-20000rfu ( 3 500 series) . If this standard cannot be reached, it can be adjusted according to the actual electrophoresis situation.
1. Configure the sample loading system according to the ratio of molecular weight internal standard : deionized formamide = 0.5μl : 9.5μl , and distribute 10μl/ well ;
2. Amount of PCR product used: 1 μl , Amount of Allelic Ladder used: 1 μl
3. After denaturation at 95°C for 3 minutes , quickly cool at 0°C for 3 minutes;
4. Electrophoresis detection: The recommended injection voltage of 3130 series is 3k Volts, and the injection time is 10 sec; the injection voltage of 3500 series is 1.2k Volts, and the injection time is 24 sec.
1. Open the Genemapper ID software , import the panels and bin files , and establish Analysis Method and Size Standard (T 500: 65, 70, 80, 100, 120, 140, 160, 180, 200, 225, 250, 275, 300, 330, 360, 390, 420, 450, 490, 500);
2. Import the electrophoresis data, select the corresponding analysis parameters such as Panel , Analysis Method and Size Standard to analyze the data.
Reagent test kit | component name | Volume (μl/tube) | Quantity (tube) |
Pre-reaction Kit | 2.5× PCR reaction buffer II | 1000 | 2 |
5× 20Aprimer mix | 500 | 2 | |
DNA Typing Standard 9947A (1ng/ μl ) | 25 | 1 | |
Deionized water Taq polymerase II | 1700 50 | 2 2 | |
Post-reaction kit | 20A Ladder | 40 | 1 |
Molecular weight internal standard T 500 | 150 | 2 |
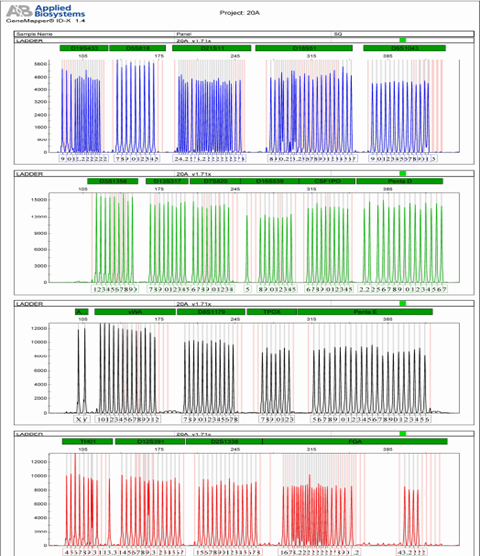
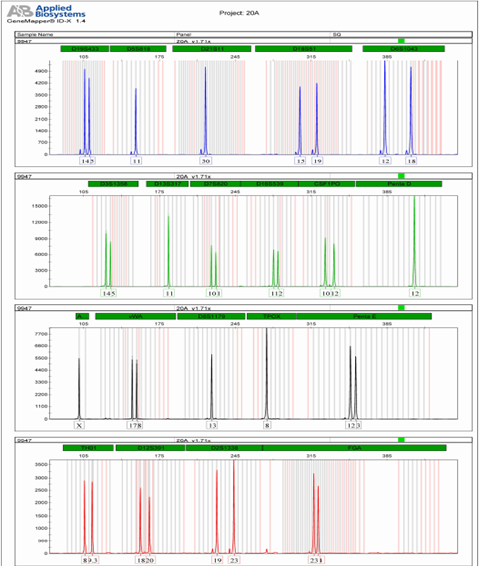
attached 2: Kit locus information
loci | Group | Control DNA | allele |
D19S433 | B | 14,15 | 9, 10, 11, 12, 12.2, 13, 13.2, 14, 14.2, 15, 15.2, 16, 16.2, 17, 17.2, |
D5S818 | B | 11,11 | 7, 8, 9, 10, 11, 12, 13, 14, 15, |
D21S11 | B | 30,30 | 24, 24.2, 25, 25.2, 26, 27, 28, 28.2, 29, 29.2, 30, 30.2, 31, 31.2, 32, 32.2, 33, 33.2, 34, 34.2, 35, 35.2, 36, 37, 38, |
D18S51 | B | 15,19 | 8, 9, 10, 10.2, 11, 12, 13, 13.2, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, |
D6S1043 | B | 12,18 | 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 21.3, |
D3S1358 | G | 14,15 | 12, 13, 14, 15, 16, 17, 18, 19, 20, |
D13S317 | G | 11,11 | 7, 8, 9, 10, 11, 12, 13, 14, 15, |
D7S820 | G | 10,11 | 6, 7, 8, 9, 10, 11, 12, 13, 14, |
D16S539 | G | 11,12 | 5, 8, 9, 10, 11, 12, 13, 14, 15, |
CSF1PO | G | 10,12 | 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, |
Penta D | G | 12,12 | 2.2, 3.2, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, |
AMEL | AND | x | X,Y, |
vWA | AND | 17.18 | 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, |
D8S1179 | AND | 13.13 | 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, |
TPOX | AND | 8.8 | 7, 8, 9, 10, 11, 12, 13, |
Penta E | AND | 12.13 | 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, |
TH01 | R. | 8,9.3 | 4, 5, 6, 7, 8, 9, 9.3, 10, 11, 13.3, |
D12S391 | R. | 18,20 | 14, 15, 16, 17, 18, 19, 19.3, 20, 21, 22, 23, 24, 25, 26, 27, |
D2S1338 | R | 19,23 | 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, |
FGA | R | 23,24 | 16, 17, 18, 18.2, 19, 19.2, 20, 20.2, 21, 21.2, 22, 22.2, 23, 23.2, 24, 24.2, 25, 25.2, 26, 27, 28, 29, 30, 31.2, 43.2, 44.2, 45.2, 46.2, |
Notice:
1. This kit is valid for one year, see the product packaging for the production date and batch number
2. Can be used for family identification, forensic research, scientific research, etc.
3. 50 servings for trial use, attached table 1 is the component list for 200 servings, and the trial pack comes with spectral correction!